
Background: Previous survival estimates in patients (pts) with polycythemia vera (PV) approach or exceed 20 y from diagnosis. However, survival in pts with PV has most often been evaluated retrospectively; as a result, granularity with respect to causes of death is often lacking. The multicenter, noninterventional, Prospective Observational Study of Patients with Polycythemia Vera in US Clinical Practices (REVEAL; NCT02252159) followed pts with PV treated in 227 community and academic practices in the US. This analysis of final data from REVEAL evaluates the characteristics of deceased pts, survival by risk, and causes of death over the course of the study.
Methods: All enrolled pts were included in this analysis. Pts were enrolled between 7/2014 and 8/2016 and were followed during usual care visits for 36 months from the date of last pt enrollment. Clinical characteristics and symptoms data were collected until death, consent withdrawal, or study end. Pts aged ≥ 60 y and/or with thrombotic event (TE) history at enrollment were classified as high-risk per modified ELN criteria. Causes of death were provided by site physicians. Descriptive statistics were used to summarize demographics, characteristics before death, and causes of death. Continuous and categorical variables were compared using t-tests and chi-square tests, respectively. Kaplan-Meier methods were used to summarize survival data. Time specific survival probabilities were compared using tests of proportions. For pts who were categorized as "alive," data were censored at the date of the last known study visit (for those lost to follow-up) or study completion.
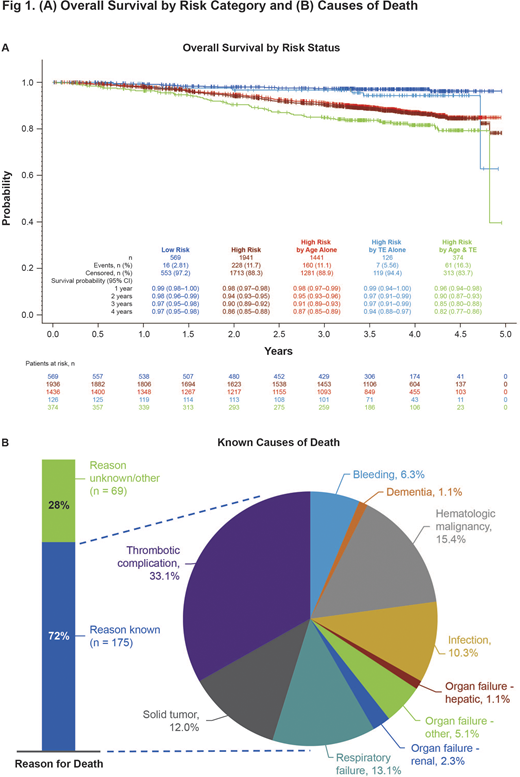
Results: Of 2510 pts enrolled (mean [SD] age, 66.3 [12.3] y); male, 54.2%), 9.7% (n=244) had died during the study and 2266 (90.3%) were alive at last known visit (mean [SD] duration of follow-up, 110.3 [58] and 179.4 [56.1] wks, respectively). Pts who had died were older at diagnosis (mean [SD] age, 68.5 [11.35] vs 60.2 [13.14] y; P < 0.001) and had numerically longer disease duration than pts who were alive (mean [SD] duration from diagnosis to enrollment, 6.5 [5.7] vs 5.7 [6.2], P = 0.06). Mean (SD) age at death was 77.1 (10.3) y with a mean (SD) disease duration at death of 8.6 (5.8) y. More pts who had died were categorized as high-risk at diagnosis compared with pts who were alive (82.0% vs 59.1%; P < 0.001), primarily due to age ≥ 60 y only (65.2% vs 45.9%; Fig. 1A). More pts who had died (vs pts who were alive) had comorbid cardiac disorders (41.0% [100/244] vs 12.1% [275/2266]), blood and lymphatic system disorders (other than PV: 31.1% [76/244] vs 18.5% [419/2266]), vascular disorders (72.1% [176/244] vs 62.3% [1412/2266]), neoplasms (47.5% [116/244] vs 22.2% [503/2266]); respiratory disorders (55.7% [136/244] vs 33.4% [757/2266]), and infections (41.0% [100/244] vs 26.3% [597/2266]; all P < 0.05). By the time of death, 34.8% (85/244) of pts had experienced a TE. In the 6 months before death, 31.1% (59/190) of pts had ≥1 elevated hematocrit (HCT) value, 57.9% (110/190) had ≥1 elevated white blood cell (WBC) count, 36.8% (70/190) had ≥1 elevated platelet count, and 27.5% (52/189) had both ≥1 elevated WBC and ≥1 elevated platelet count (ie, uncontrolled myeloproliferation).
The survival probability (95% CI) in all pts was 98% (0.98-0.99) at 1-y and 89% (0.87-0.90) at 4-y post-enrollment. The survival probability at 4 y was lower for high- vs low-risk pts (86% [0.85-0.88] vs 97% [0.95-0.98]; P < 0.001; Fig. 1A). Among high-risk pts, 4-y survival was 82% for pts aged >60 y and with TE history, 87% for pts aged >60 y alone, and 94% for pts with TE history alone. Of pts with known cause of death (n=175), the most common cause was thrombotic complications (33.1%; Fig. 1B).
Conclusions: In this analysis from REVEAL, the largest prospective and contemporary cohort of pts with PV in the US, the estimated 4-y mortality was >10%, a finding that is surprising given the mean age at enrollment was only ~66 y. Approximately one-third of the deaths were due to thrombotic complications; in the 6 months prior to death, more than a quarter of pts had elevated HCT or uncontrolled myeloproliferation. Compared with pts alive at study completion, pts who had died had higher-risk disease, and higher rates of comorbid conditions. The high rate of respiratory disorders observed in the deceased population, both as comorbidities and causes of death, has not been well characterized in other PV mortality studies and warrants further investigation.
Stein:Kartos: Other: educational content presented; Constellation Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pharmaessentia: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding. Scherber:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Yu:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Paranagama:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Miller:Incyte: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal